* [[Pimethixene]]<ref>{{cite web | title=Insecticidal tricyclic derivatives | website=Google Patents | date=2003-09-12 | url=https://patents.google.com/patent/WO2004026030A2/en?oq=WO2004026030 | access-date=2025-06-11}}</ref>
* [[Pimethixene]]<ref>{{cite web | title=Insecticidal tricyclic derivatives | website=Google Patents | date=2003-09-12 | url=https://patents.google.com/patent/WO2004026030A2/en?oq=WO2004026030 | access-date=2025-06-11}}</ref>
* [[Piperylone]]<ref>{{cite patent |country=US |number=2903460 |inventor = Jucker E, Ebnother A, Lindenmann AJ |title=Pyrazolone derivatives |status=patent |gdate=1959-09-08 |fdate=1958-12-09 |assign1=Sandoz AG}}</ref><ref>{{cite journal |doi=10.1002/hlca.19590420415 |title=Über neuartige, basisch substituierte Pyrazolon-Derivate. Untersuchungen über synthetische Arzneimittel. 3. Mitteilung |date=1959 | vauthors = Ebnöther A, Jucker E, Lindenmann A |journal=Helvetica Chimica Acta |volume=42 |issue=4 |pages=1201–1214 }}</ref><ref>{{cite web |url=https://pharmaceutical-substances.thieme.com/ps/search-results?docUri=KD-16-0123 |title=Piperylone |work=Pharmaceutical Substances |publisher=Thieme |access-date=2024-07-17 }}</ref>
* [[Piperylone]]<ref>{{cite patent |country=US |number=2903460 |inventor = Jucker E, Ebnother A, Lindenmann AJ |title=Pyrazolone derivatives |status=patent |gdate=1959-09-08 |fdate=1958-12-09 |assign1=Sandoz AG}}</ref><ref>{{cite journal |doi=10.1002/hlca.19590420415 |title=Über neuartige, basisch substituierte Pyrazolon-Derivate. Untersuchungen über synthetische Arzneimittel. 3. Mitteilung |date=1959 | vauthors = Ebnöther A, Jucker E, Lindenmann A |journal=Helvetica Chimica Acta |volume=42 |issue=4 |pages=1201–1214 }}</ref><ref>{{cite web |url=https://pharmaceutical-substances.thieme.com/ps/search-results?docUri=KD-16-0123 |title=Piperylone |work=Pharmaceutical Substances |publisher=Thieme |access-date=2024-07-17 }}</ref>
*[[RS-86]] [3576-73-6] Fumarate salt: [51382-46-8]<ref>{{cite journal | vauthors=((Ghose, K.)) | journal=Drugs of the Future | title=RS-86 | volume=11 | issue=4 | pages=276 | date= 1986 | url=http://journals.prous.com/journals/servlet/xmlxsl/pk_journals.xml_summary_pr?p_JournalId=2&p_RefId=65133&p_IsPs=N | issn=0377-8282 | doi=10.1358/dof.1986.011.04.65133}}</ref><ref>{{cite journal | vauthors=((Cignarella, G.)), ((Villa, S.)), ((Cattabeni, F.)), ((Renò, F.)), ((Cimino, M.)), ((De Benedetti, P.)), ((Barlocco, D.)) | journal=European Journal of Medicinal Chemistry | title=Synthesis of a new series of 2,8-disubstituted-2,8-diazaspiro[4,5]decan-1-ones as potential muscarinic agonists | volume=29 | issue=12 | pages=955–961 | date= January 1994 | url=https://linkinghub.elsevier.com/retrieve/pii/0223523494901953 | doi=10.1016/0223-5234(94)90195-3}}</ref>
*[[SN-22]]<ref>{{cite journal | last1=Cole | first1=Derek C. | last2=Ellingboe | first2=John W. | last3=Lennox | first3=William J. | last4=Mazandarani | first4=Hossein | last5=Smith | first5=Deborah L. | last6=Stock | first6=Joseph R. | last7=Zhang | first7=Guoming | last8=Zhou | first8=Ping | last9=Schechter | first9=Lee E. | title=N1-arylsulfonyl-3-(1,2,3,6-tetrahydropyridin-4-yl)-1H-indole derivatives are potent and selective 5-HT6 receptor antagonists | journal=Bioorganic & Medicinal Chemistry Letters | publisher=Elsevier BV | volume=15 | issue=2 | year=2005 | issn=0960-894X | doi=10.1016/j.bmcl.2004.10.064 | pages=379–383| pmid=15603958 }}</ref>
*[[SN-22]]<ref>{{cite journal | last1=Cole | first1=Derek C. | last2=Ellingboe | first2=John W. | last3=Lennox | first3=William J. | last4=Mazandarani | first4=Hossein | last5=Smith | first5=Deborah L. | last6=Stock | first6=Joseph R. | last7=Zhang | first7=Guoming | last8=Zhou | first8=Ping | last9=Schechter | first9=Lee E. | title=N1-arylsulfonyl-3-(1,2,3,6-tetrahydropyridin-4-yl)-1H-indole derivatives are potent and selective 5-HT6 receptor antagonists | journal=Bioorganic & Medicinal Chemistry Letters | publisher=Elsevier BV | volume=15 | issue=2 | year=2005 | issn=0960-894X | doi=10.1016/j.bmcl.2004.10.064 | pages=379–383| pmid=15603958 }}</ref>
*[[Tubastatin A]] [1252003-15-8] TFA salt: [1239262-52-2]<ref>{{cite journal | vauthors=((Butler, K. V.)), ((Kalin, J.)), ((Brochier, C.)), ((Vistoli, G.)), ((Langley, B.)), ((Kozikowski, A. P.)) | journal=Journal of the American Chemical Society | title=Rational Design and Simple Chemistry Yield a Superior, Neuroprotective HDAC6 Inhibitor, Tubastatin A | volume=132 | issue=31 | pages=10842–10846 | date=11 August 2010 | url=https://pubs.acs.org/doi/10.1021/ja102758v | doi=10.1021/ja102758v}}</ref>
*[[Tubastatin A]] [1252003-15-8] TFA salt: [1239262-52-2]<ref>{{cite journal | vauthors=((Butler, K. V.)), ((Kalin, J.)), ((Brochier, C.)), ((Vistoli, G.)), ((Langley, B.)), ((Kozikowski, A. P.)) | journal=Journal of the American Chemical Society | title=Rational Design and Simple Chemistry Yield a Superior, Neuroprotective HDAC6 Inhibitor, Tubastatin A | volume=132 | issue=31 | pages=10842–10846 | date=11 August 2010 | url=https://pubs.acs.org/doi/10.1021/ja102758v | doi=10.1021/ja102758v}}</ref>
Chemical compound
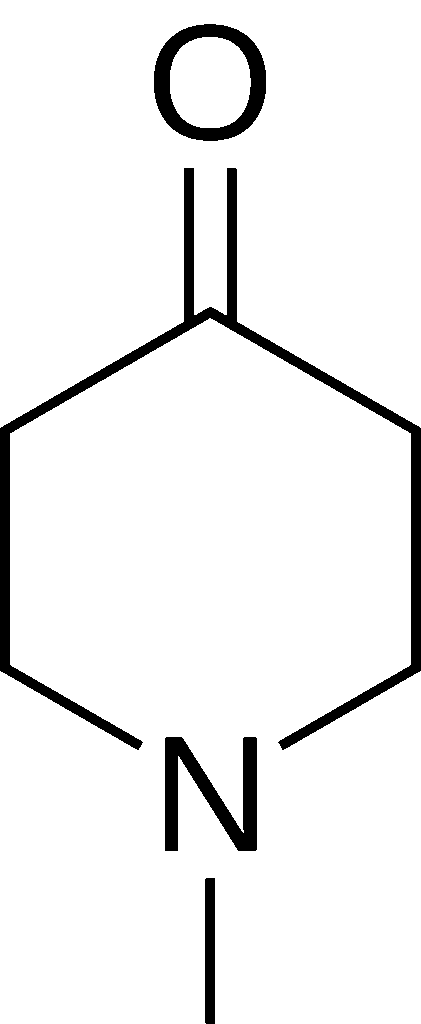
1-Methyl-4-piperidone (also known as N-methyl-4-piperidone) is a clear dark yellow liquid organic compound with molecular formula C6H11NO. It is the N-methyl substituted variant of 4-piperidone.[1]
It is used as a building block for a variety of pharmaceuticals including:
The first synthesis of 1-methyl-4-piperidone was reported by Samuel M. McElvain in 1948.[25] The method involves double Michael reaction between methylamine and two equivalents of ethyl acrylate, a Dieckmann cyclization (i.e. intramolecular Claisen condensation), saponification and decarboxylation.
Another synthesis uses methylamine, formaldehyde and the ethyl ester of acetonedicarboxylic acid.[26]
A third synthesis consists of the ring closing of 1,5-dichloro-3-pentanone with methylamine.[27]
- ^ “SAFETY DATA SHEET”. Retrieved 2025-06-11.
- ^ “Azacyclic compounds for use in the treatment of serotonin related diseases”. Google Patents. March 6, 2001. Retrieved June 27, 2025.
- ^ Eistetter K, Kley HP, Menge HG, Schaefer H (1983). “B-777-81”. Drugs of the Future. 8 (5): 387. doi:10.1358/dof.1983.008.05.65796.
- ^ “Anti-depressant and analgesic 4-phenoxypiperidines”. Google Patents. July 11, 1980. Retrieved June 11, 2025.
- ^ “4-Aryl-4-aryloxypiperidines”. Google Patents. September 3, 1980. Retrieved June 11, 2025.
- ^ “X-tertiary amino-i-alkyl-piperidines”. Google Patents. July 13, 1954. Retrieved June 11, 2025.
- ^ Bandyopadhyaya, Achintya; Rajagopalan, Desikan R.; Rath, Nigam P.; Herrold, Amy; Rajagopalan, Raghavan; Napier, T. Celesete; Tedford, Clark E.; Rajagopalan, Parthasarathi (2012). “The synthesis and receptor binding affinities of DDD-016, a novel, potential, atypical antipsychotic”. MedChemComm. 3 (5): 580. doi:10.1039/c2md00311b. ISSN 2040-2503. Retrieved 2025-06-11.
- ^ van Der Stelt, C.; Harms, A. F.; Nauta, W. Th. (1961). “The Effect of Alkyl-Substitution in Drugs–V. Synthesis and Chemical Properties of Some Dibenzo [a,d]1,4-cycloheptadienyl Ethers”. Journal of Medicinal and Pharmaceutical Chemistry. 4 (2): 335–349. doi:10.1021/jm50018a008. ISSN 0095-9065. PMID 14039500. Retrieved 2025-06-26.
- ^ Filla, Sandra A.; Mathes, Brian M.; Johnson, Kirk W.; Phebus, Lee A.; Cohen, Marlene L.; Nelson, David L.; Zgombick, John M.; Erickson, Jon A.; Schenck, Kathryn W.; Wainscott, David B.; Branchek, Theresa A.; Schaus, John M. (2003-07-01). “Novel Potent 5-HT 1F Receptor Agonists: Structure−Activity Studies of a Series of Substituted N -[3-(1-Methyl-4-piperidinyl)-1 H -pyrrolo[3,2- b ]pyridin-5-yl]amides”. Journal of Medicinal Chemistry. 46 (14): 3060–3071. doi:10.1021/jm030020m. ISSN 0022-2623. PMID 12825944. Retrieved 2025-06-11.
- ^ Hörlein, Ulrich (1954). “Zur Kenntnis der Tetrahydrocarbolin-Verbindungen (I. Mitteil.)”. Chemische Berichte. 87 (4): 463–472. doi:10.1002/cber.19540870404. ISSN 0009-2940. Retrieved 2025-06-11.
- ^ “Preparation method of mebhydrolin napadisylate”. Google Patents. 2013-01-31. Retrieved 2025-06-11.
- ^ “Process for preparation of naratriptan hydrochloride”. Google Patents. 2009-03-09. Retrieved 2025-06-11.
- ^ “A process for the synthesis of naratriptan”. Google Patents. 2010-08-18. Retrieved 2025-06-11.
- ^ “Molecule of the Month August 2023”. School of Chemistry. 2016-08-18. Retrieved 2025-06-11.
- ^ “Process for preparation of substituted formamidine and substituted N-iminomethyl piperidine”. Google Patents. 1983-03-17. Retrieved 2025-06-11.
- ^ “Deuterated pimavanserin 1- (4-flu0r0benzyl) -3- (4-isobutoxybenzyl) -1- ( l-methyl-piperidin-4-yl) -urea”. Google Patents. May 8, 2008. Retrieved June 27, 2025.
- ^ “Insecticidal tricyclic derivatives”. Google Patents. 2003-09-12. Retrieved 2025-06-11.
- ^ US patent 2903460, Jucker E, Ebnother A, Lindenmann AJ, “Pyrazolone derivatives”, issued 1959-09-08, assigned to Sandoz AG
- ^ Ebnöther A, Jucker E, Lindenmann A (1959). “Über neuartige, basisch substituierte Pyrazolon-Derivate. Untersuchungen über synthetische Arzneimittel. 3. Mitteilung”. Helvetica Chimica Acta. 42 (4): 1201–1214. doi:10.1002/hlca.19590420415.
- ^ “Piperylone”. Pharmaceutical Substances. Thieme. Retrieved 2024-07-17.
- ^ Ghose, K. (1986). “RS-86”. Drugs of the Future. 11 (4): 276. doi:10.1358/dof.1986.011.04.65133. ISSN 0377-8282.
- ^ Cignarella, G., Villa, S., Cattabeni, F., Renò, F., Cimino, M., De Benedetti, P., Barlocco, D. (January 1994). “Synthesis of a new series of 2,8-disubstituted-2,8-diazaspiro[4,5]decan-1-ones as potential muscarinic agonists”. European Journal of Medicinal Chemistry. 29 (12): 955–961. doi:10.1016/0223-5234(94)90195-3.
- ^ Cole, Derek C.; Ellingboe, John W.; Lennox, William J.; Mazandarani, Hossein; Smith, Deborah L.; Stock, Joseph R.; Zhang, Guoming; Zhou, Ping; Schechter, Lee E. (2005). “N1-arylsulfonyl-3-(1,2,3,6-tetrahydropyridin-4-yl)-1H-indole derivatives are potent and selective 5-HT6 receptor antagonists”. Bioorganic & Medicinal Chemistry Letters. 15 (2). Elsevier BV: 379–383. doi:10.1016/j.bmcl.2004.10.064. ISSN 0960-894X. PMID 15603958.
- ^ Butler, K. V., Kalin, J., Brochier, C., Vistoli, G., Langley, B., Kozikowski, A. P. (11 August 2010). “Rational Design and Simple Chemistry Yield a Superior, Neuroprotective HDAC6 Inhibitor, Tubastatin A”. Journal of the American Chemical Society. 132 (31): 10842–10846. doi:10.1021/ja102758v.
- ^ McElvain, S. M., Rorig, K. (May 1948). “Piperidine Derivatives. XVIII. The Condensation of Aromatic Aldehydes with 1-Methyl-4-piperidone”. Journal of the American Chemical Society. 70 (5): 1820–1825. Bibcode:1948JAChS..70.1820M. doi:10.1021/ja01185a051. PMID 18861789.
- ^ “A kind of synthetic method of N- methyl -4- piperidones”. Google Patents. September 3, 2019. Retrieved June 11, 2025.
- ^ “Synthesis method for N-substituted-4-piperidone”. Google Patents. May 24, 2012. Retrieved June 11, 2025.