{{Short description|Popular science overview of quantum mechanics}}
”’Quantum: A Walk Through The Universe”’
{{Use dmy dates|date=September 2025}}
[[File:Quantum physics.jpg|thumb|60px|Entanglement]]
{{More citations needed|date=September 2025}}
Quantum science describes the behaviour of matter and light on the atomic and subatomic scale,” intones the introduction to the National Quantum Strategy.
”’Quantum: A Walk Through the Universe”'<br> ”A Frivolous Approach to Quantum”<br>
Atoms, matter, and light. Easy. But then:
Is a popular science overview of [[quantum mechanics]], its history, central concepts, and emerging technologies. Written in an informal style, the work introduces principles such as [[quantisation]], [[superposition]], and [[quantum entanglement]], while linking them to both foundational experiments and modern applications.
[[File:Quantum physics.jpg|thumb|upright=0.5|Conceptual illustration of entanglement]]
“Quantum behaviours – particularly quantisation, superposition and entanglement – can be used to build advanced technologies that would otherwise seem impossible,” continues the Strategy.
== Overview ==
Look up quantisation, superposition, and entanglement, and you will be met with a barrage of complicated and contradictory explanations. Quantum science has very much earned its reputation for being difficult to understand, and no Strategy can lay it out with perfect neatness.
Quantum mechanics describes the behaviour of matter and light at the atomic and subatomic scale.<ref name=”NQS”>National Quantum Strategy (2023).</ref> These phenomena underlie technologies such as [[Semiconductor|semiconductors]], [[Laser|lasers]], and [[Solar cell|solar cells]], and form the basis of developing fields including [[Quantum computing|quantum computing]] and [[Quantum sensor|quantum sensing]].<ref name=”White”>White, Andrew. ARC Centre of Excellence for Engineered Quantum Systems.</ref>
“It is a very precise theory framework about how the world works,” says Professor Stephen Bartlett, a quantum physicist and director of the University of Sydney’s Nano Institute.
theory the , University of Nano Institute.
== Historical background ==
“What makes it tricky and conceptually challenging is that very precise theory is completely at odds with our conventional understanding, based on our intuitive interaction with the world.”
=== Early challenges to classical physics ===
In the 19th century, classical physics described motion, gravity, and electromagnetism with high precision. Experiments such as [[Thomas Young]]’s 1801 [[double-slit experiment]] supported the wave theory of light.<ref>Young, Thomas (1801). “On the Nature of Light and Colours”.</ref>
At the turn of the 20th century, anomalies emerged. The [[photoelectric effect]] showed that increasing light intensity did not increase electron kinetic energy as wave theory predicted. [[Max Planck]] (1900) proposed that light was emitted in discrete packets, or ”quanta”.<ref>Planck, Max (1900). “On the Theory of the Energy Distribution Law of the Normal Spectrum”.</ref> [[Albert Einstein]] expanded this idea in 1905, introducing the concept of the [[photon]].<ref>Einstein, Albert (1905). “On a Heuristic Viewpoint Concerning the Production and Transformation of Light”.</ref>
So perhaps, to get our heads around quantum science, we should begin with some old conventional understandings.
[[Niels Bohr]]’s 1913 atomic model explained electron behaviour in hydrogen but was limited for larger atoms and molecules. By 1927, Clinton Davisson and Lester Germer demonstrated electron diffraction, providing direct evidence of [[wave–particle duality]].
A deeply wrong intuition
The problem with wrong science is that it often feels intuitively right. I, for instance, have always thought of atoms as tiny immutable balls. These little spheres interact with each other to become molecules, and all the stuff I can feel and see and taste.
=== Schrödinger’s equation ===
If I concentrated, I might picture slightly smaller balls inside the atoms – protons, neutrons, and electrons – but I didn’t delve more deeply than that. I knew this model was wrong, but it was sufficient for understanding what I needed and wanted to understand.
In 1925–26, [[Erwin Schrödinger]] formulated the [[Schrödinger equation]], describing the probabilistic behaviour of quantum systems through the wavefunction (ψ). Debate persists on whether the wavefunction represents physical reality or knowledge of a system.<ref>Schrödinger, Erwin (1926). “Quantisation as an Eigenvalue Problem”.</ref> The equation enables prediction of atomic and molecular structures and underpins semiconductor physics.
== Key concepts ==
Quantum physics shatters the spheres around atoms, protons, neutrons, and electrons. It shatters the borders between numbers and reality, between form and matter, between thing and action.
=== Measurement and uncertainty ===
[[Werner Heisenberg]]’s 1927 [[uncertainty principle]] formalised the limitations of measuring quantum systems, in which observation itself alters the system. This led to probabilistic rather than deterministic outcomes.
=== Superposition ===
“We learn how the world works on our length scale and our time scale, but that’s not how the world works when you get to the very small and the very fast,” says Professor Andrew White, a quantum physicist at The University of Queensland and director of the ARC Centre of Excellence for Engineered Quantum Systems.
Superposition refers to the ability of quantum systems to exist in multiple states simultaneously until measurement. Schrödinger illustrated the paradox with the 1935 [[Schrödinger’s cat]] thought experiment.
=== Entanglement ===
“There’s different rules there, but we have no intuitive sense of those rules.”
[[Quantum entanglement]] occurs when particles share correlated states such that measurement of one instantaneously influences the other, regardless of distance. Einstein criticised this as “spooky action at a distance” in the [[EPR paradox]], but later experiments confirmed the effect.<ref>Bell, J. S. (1964). “On the Einstein Podolsky Rosen paradox”. Physics Physique Физика.</ref> Entanglement is now central to [[quantum cryptography]] and related technologies.
== Applications ==
But these counterintuitive rules work.
Quantum mechanics underpins 20th-century technologies such as transistors, lasers, and magnetic resonance imaging. A “second quantum revolution” is under way, exploiting superposition and entanglement for new applications, including:
* ”’Quantum computing”’: using [[qubit]]s for information processing.
* ”’Quantum sensing”’: ultraprecise sensors, including devices capable of operating without [[Global Positioning System|GPS]].
* ”’Quantum communication”’: secure communication enabled by entanglement.
* ”’Optical clocks”’: highly precise quantum timekeeping, including devices developed at the [[University of Adelaide]].
Australian research institutions, including the University of Sydney, the University of Queensland, and the University of Adelaide, are noted contributors to international quantum research.<ref name=”Monro”>Monro, Tanya. Chief Defence Scientist, Australian Department of Defence.</ref>
“It’s the most successful theory we have of how the world works. We’ve got huge faith in it. There’s huge repeatability,” says White.
== Interpretations ==
The problem with light
While quantum mechanics is experimentally well verified, its interpretation remains contested. Some physicists view the wavefunction as an element of physical reality, while others regard it as a tool for predicting measurement outcomes.<ref>Zeilinger, Anton. Nobel Lecture (2022).</ref>
The first kernels of quantum science emerged at the turn of the 20th Century. By this point, physicists were very confident about the rules for gravity, light and matter. These rules, now termed ‘classical physics’, worked well for everything we can see – from the pen sliding off your notebook, to the moons
of Jupiter.
== See also ==
Physicists thought they had established that light was a wave. It emanated out from a source of energy, like ripples of water coming from a stone dropped in a pond.
* [[History of quantum mechanics]]
* [[Wave–particle duality]]
* [[Schrödinger equation]]
* [[Quantum entanglement]]
* [[Quantum computing]]
== References ==
One of the more successful proofs had been Thomas Young’s 1801 double slit experiment. If light is shone on a plate containing 2 slits, it will appear in a pretty, striped pattern on a screen on the other side.
{{Reflist}}
“If I want to drop 2 rocks [in water] at the same time, I get really interesting patterns, where in some places the water is completely flat, and other places get super deep or super high waves. We call that an interference pattern,” explains White.
[[Category:Quantum mechanics]]
If light is a wave, surely more energy causes bigger waves. An anvil tossed into a pond is going to cause bigger ripples than a marble. But experiments weren’t showing this held true with light. For instance, if you shine light on a metal, it can eject electrons. Higher intensity light should cause higher kinetic energy in the ejected electrons, but it doesn’t – it just causes more electrons to be ejected.
[[Category:Popular physics books]]
[[Category:History of physics]]
Max Planck sought to explain this in 1900 by suggesting that light waves worked in chunks of energy: more energy, and you get more chunks, not bigger waves. He called these chunks ‘quanta’, which means how much in Latin.
Albert Einstein expanded on this idea in one of his 4 sphere-shattering 1905 papers (the other 3 were busy revolutionising other parts of physics). The concept grew into the ‘photon’: the light particle. While light can work in waves, it can also work in photons – experiments can tell you either.
Bohr’s 1913 model was a leap in understanding matter: it could successfully describe the way electrons behaved inside atoms. It matched experimental data brilliantly for small, individual atoms, but there were other observations about matter that it couldn’t explain – like molecules. It took another 10 years of mounting contradictions to understand why.
By 1925, several physicists were beginning to circle around the same answer: if light waves can behave like particles, could the reverse also be true? Could particles – like electrons – behave like waves?
Yes. There were a few different ways of showing it, but here’s a familiar one: In 1927, Clinton Davisson and Lester Germer tried the double-slit experiment with a beam of electrons.
“You could make a prediction based on classical physics about what you’ll see on the other side of that wall, and that is a pile up of splatter behind one of the slits and a pile up of splatter behind the other slit,” says Bartlett.
And if electrons were particles, this is the result you would see. But.
“If you actually do this with atoms or electrons, what you see is very much like what you saw when you did it with light – an interference pattern,” says Bartlett.
“Wave-particle duality is the idea that at the nanoscale, things that we normally think of as chunks can behave like waves – like atoms. Things that we normally think of as waves, like light, can behave like chunks,” summarises White.
“All of the revolutions in technology in semiconductors, solar cells, the second half the 20th century and the first part of this century – that’s all one piece of quantum physics, which is the wave-particle duality.”
“To understand quantum, it helps to have a really good understanding of mathematics.”
A wavefunction solution
There are several different ways to fold this wave-particle duality into our understanding of matter. Erwin Schrödinger penned the most successful in 1925. Published in 1926, it’s now called the Schrödinger Equation:
Schrödinger’s equation
Each of those elegant symbols represent some far more complicated mathematics: differentials, operators, derivatives… You can solve this equation, but you can’t solve it with high school algebra.
“We get used to using maths as one of our second languages. To understand quantum, it helps to have a really good understanding of mathematics as well,” says White.
For those of us learning the language, the most crucial term in there is ψ – the Greek letter psi. In quantum physics, ψ represents a thing called the wavefunction.
“Physicists can’t even agree amongst themselves: is it an element of reality? Is it a real thing that happens, or is it just a description of our state of knowledge?” says White. He’s in the former camp, recent Nobel laureate Anton Zeilinger is in the latter.
“He’s got a Nobel Prize and I don’t. So what do I know? But still, I think I’m right,” quips White.
Whatever its true identity, working with ψ can give you real results.
“It tells you the probability of the system being here or there. What it doesn’t tell you is the system will definitely be here or there. It’s all probability,” says White.
Solve the Schrödinger equation for a given system, and you can predict the system’s properties. You can learn where electrons are likely to be in a molecule, and thus its shape, or how a semiconductor might semi-conduct. Any device with a transistor in it is using this quantum physics to work.
We have become very familiar with the wave behaviour of particles over the past century. But that’s not the only concept that Schrödinger’s equation embraces. Those 20th-century physicists made a few other crucial observations that are now crossing from theory into engineering.
“It’s those extra capabilities that are driving this so-called second quantum revolution that we’re living through,” says White.
So what’s making waves now?
Quantum measurement
“When you have these very small systems, if you look at them, the act of observing changes the thing that you’re looking at,” says White.
This concept, articulated by Werner Heisenberg’s 1927 Uncertainty Principle, has very practical roots.
“Think of what it means to observe something,” prompts Bartlett. In a chemistry lab, for instance, you might observe that a reaction has happened because liquid in a beaker has turned from colourless to bright pink.
In watching that beaker, you are in fact interacting with it – light is bouncing off the beaker, and travelling into your eyeball. A huge number of photons are giving your eye the ‘pink’ signal.
What if you’re studying the photons themselves? Or electrons, or other particles that are similarly tiny? You could swap your eyes out for more delicate equipment, but ultimately, something has to hit a detector for you to know what’s going on.
“[It’s] this idea of quantisation: you can’t just make your light go as dim as you want. At some point, you’re actually shining a single photon, which is a single particle of light, on that object, and you can’t have any less than that,” says Bartlett.
In the double-slit experiment, we know electrons were behaving as waves because of the way they hit the screen on the other side. But when each electron hits the screen, it does it in a discrete, particle-like point. Study an individual photon or electron, and while you know it has wave-like behaviour, hitting a detector will give you an individual value.
“It’s a fun exercise we do with our students. Think of other ways that you can sneakily observe or infer something about a quantum system,” says Bartlett.
“You always, in the end, come down to some sort of interaction.”
Superposition
Both Schrödinger’s equation and the uncertainty around measurement prompt the idea of superposition.
“Superposition is the fact that – I don’t want to say things can be in 2 places at once, but you can have no knowledge of which place the thing is in,” says White.
“You have to treat it like it could be in either place simultaneously.”
Schrödinger might have written the equation that underpins most of quantum mechanics.
But chances are that if you recognised his name, it was because of a deliberately silly provocation he came up with 1935 to emphasise the absurdity of superposition.
Schrödinger’s Cat is a thought experiment: imagine a cat trapped in a box with a vial of poison and a radioactive substance that may or may not decay. If it decays, it will prompt a Geiger counter to break the poison vial and kill the cat.
The radioactive substance is a quantum object: you can run it through the Schrödinger equation and come up with answers that it both will and won’t decay – you won’t know which until it’s been observed.
What does this mean for the poor cat?
Physicists have come up with a variety of answers to this paradox, none universally agreed upon and none entirely satisfying. The cat problem hints at bigger problems with the meaning of quantum
science. But working with the assumption of a superimposed state, where both possibilities could be true until you measure them, still gives us accurate answers.
In it, they pointed out that a conclusion of all these quantum calculations was that 2 particles could interact such that their wavefunctions couldn’t be split apart. So even if the particles were separated over a great distance, measuring one would tell you something about the other. This violated one of Einstein’s other well-established theories: that things cannot travel faster than the speed of light.
It was Schrödinger who coined the term “entanglement”, in a response to the EPR Paradox. Debate raged over whether entanglement was an impossible idea – Einstein termed it “spooky action at a distance” and used it to suggest that there was something wrong with quantum theory – but physicists have since proved its existence experimentally.
“Entanglement is correlation between quantum systems that really defies common sense,” says White.
“There’s no easy set of rules I can give you that will show you how these systems will be correlated.”
But we know that it happens – in fact, it’s already being used in quantum cryptography.
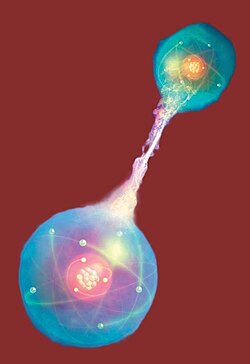
Two blue particles interferring with each other, looking like an energy zap.
Conceptual artwork of a pair of entangled quantum particles or events (left and right) interacting at a distance. Credit: Mark Garlick/Science Photo Library, Getty Images.
New intuitions
“Quantum is not magic,” says White.
“It does wonderful, surprising, engaging, powerful things, but it’s not magic. We know the rules really well. What’s interesting about quantum is there’s not yet a universally agreed upon interpretation of what the rules mean.”
The unclear meaning has not stopped people from building things.
“We know how it works. Why it works is a really fun separate question, but we don’t need to answer it,” says White.
Flip though the National Quantum Strategy, and you will find a bevy of impossible technologies: high-power sensors, super-fast energy storage, computers that can calculate exactly how a new drug might interact with a human body.
“We’re pretty sure it’s going to transform every sector of the economy that it touches,” says White.
“I don’t think what is well known in Australia is that, in terms of quantum physics, for the last quarter of a century or more, we have been world leaders.”
The defence industry is already capitalising on quantum. Professor Tanya Monro, Chief Defence Scientist, tells me quantum sensors could work in lieu of GPS when satellite connection is shaky.
“By harnessing the quantum characteristics of atoms, we can make sensors that are exquisitely sensitive,” she says. Australian quantum tech won out at the most recent Rim of the Pacific exercise, where nations friendly to the USA rattle their swords.
“The Australian optical clocks out of the University of Adelaide gave evidence of world’s best quantum clock performance,” says Monro. Then there’s quantum communications – “obviously communication is a critical element of any modern military system,” she adds.
Bartlett is excited by quantum computing – quantum bits, or qubits, wrapped together to provide extraordinary information processing power. Proof-of-concept quantum computers already exist, but usable ones are a handful of years away.
“If you read science fiction stories or the press from the early part of the 20th century, the way they talked about magnetism and electricity is a little bit how we talk about quantum now,” says White.
Once, the idea that you could get light by flicking a switch was extraordinary. “Now it’s just a commonplace miracle, and no one thinks anything of it,” says White.
“I think that’s where we’ll be in another generation or two with quantum. But right now, it’s weird as.”
Popular science overview of quantum mechanics
Quantum: A Walk Through the Universe
A Frivolous Approach to Quantum
Is a popular science overview of quantum mechanics, its history, central concepts, and emerging technologies. Written in an informal style, the work introduces principles such as quantisation, superposition, and quantum entanglement, while linking them to both foundational experiments and modern applications.

Quantum mechanics describes the behaviour of matter and light at the atomic and subatomic scale.[1] These phenomena underlie technologies such as semiconductors, lasers, and solar cells, and form the basis of developing fields including quantum computing and quantum sensing.[2]
Physicists have described quantum mechanics as both the most successful theory of nature and one of the most conceptually challenging, as its principles often conflict with intuitive human experience.[3]
Historical background
[edit]
Early challenges to classical physics
[edit]
In the 19th century, classical physics described motion, gravity, and electromagnetism with high precision. Experiments such as Thomas Young’s 1801 double-slit experiment supported the wave theory of light.[4]
At the turn of the 20th century, anomalies emerged. The photoelectric effect showed that increasing light intensity did not increase electron kinetic energy as wave theory predicted. Max Planck (1900) proposed that light was emitted in discrete packets, or quanta.[5] Albert Einstein expanded this idea in 1905, introducing the concept of the photon.[6]
Niels Bohr’s 1913 atomic model explained electron behaviour in hydrogen but was limited for larger atoms and molecules. By 1927, Clinton Davisson and Lester Germer demonstrated electron diffraction, providing direct evidence of wave–particle duality.
Schrödinger’s equation
[edit]
In 1925–26, Erwin Schrödinger formulated the Schrödinger equation, describing the probabilistic behaviour of quantum systems through the wavefunction (ψ). Debate persists on whether the wavefunction represents physical reality or knowledge of a system.[7] The equation enables prediction of atomic and molecular structures and underpins semiconductor physics.
Measurement and uncertainty
[edit]
Werner Heisenberg’s 1927 uncertainty principle formalised the limitations of measuring quantum systems, in which observation itself alters the system. This led to probabilistic rather than deterministic outcomes.
Superposition refers to the ability of quantum systems to exist in multiple states simultaneously until measurement. Schrödinger illustrated the paradox with the 1935 Schrödinger’s cat thought experiment.
Quantum entanglement occurs when particles share correlated states such that measurement of one instantaneously influences the other, regardless of distance. Einstein criticised this as “spooky action at a distance” in the EPR paradox, but later experiments confirmed the effect.[8] Entanglement is now central to quantum cryptography and related technologies.
Quantum mechanics underpins 20th-century technologies such as transistors, lasers, and magnetic resonance imaging. A “second quantum revolution” is under way, exploiting superposition and entanglement for new applications, including:
- Quantum computing: using qubits for information processing.
- Quantum sensing: ultraprecise sensors, including devices capable of operating without GPS.
- Quantum communication: secure communication enabled by entanglement.
- Optical clocks: highly precise quantum timekeeping, including devices developed at the University of Adelaide.
Australian research institutions, including the University of Sydney, the University of Queensland, and the University of Adelaide, are noted contributors to international quantum research.[9]
While quantum mechanics is experimentally well verified, its interpretation remains contested. Some physicists view the wavefunction as an element of physical reality, while others regard it as a tool for predicting measurement outcomes.[10]
- ^ National Quantum Strategy (2023).
- ^ White, Andrew. ARC Centre of Excellence for Engineered Quantum Systems.
- ^ Bartlett, Stephen. University of Sydney Nano Institute.
- ^ Young, Thomas (1801). “On the Nature of Light and Colours”.
- ^ Planck, Max (1900). “On the Theory of the Energy Distribution Law of the Normal Spectrum”.
- ^ Einstein, Albert (1905). “On a Heuristic Viewpoint Concerning the Production and Transformation of Light”.
- ^ Schrödinger, Erwin (1926). “Quantisation as an Eigenvalue Problem”.
- ^ Bell, J. S. (1964). “On the Einstein Podolsky Rosen paradox”. Physics Physique Физика.
- ^ Monro, Tanya. Chief Defence Scientist, Australian Department of Defence.
- ^ Zeilinger, Anton. Nobel Lecture (2022).