EML
| ← Previous revision | Revision as of 06:14, 15 September 2025 | ||
| Line 1: | Line 1: | ||
|
{{Short description|Chemical compound}}
|
{{Short description|Chemical compound}}
|
||
|
{{Use dmy dates|date=September 2025}}
|
|||
|
{{Drugbox
|
|||
|
{{cs1 config|name-list-style=vanc|display-authors=6}}
|
|||
|
{{Infobox drug
|
|||
|
| Verifiedfields = changed
|
| Verifiedfields = changed
|
||
|
| Watchedfields = changed
|
| Watchedfields = changed
|
||
| Line 6: | Line 8: | ||
|
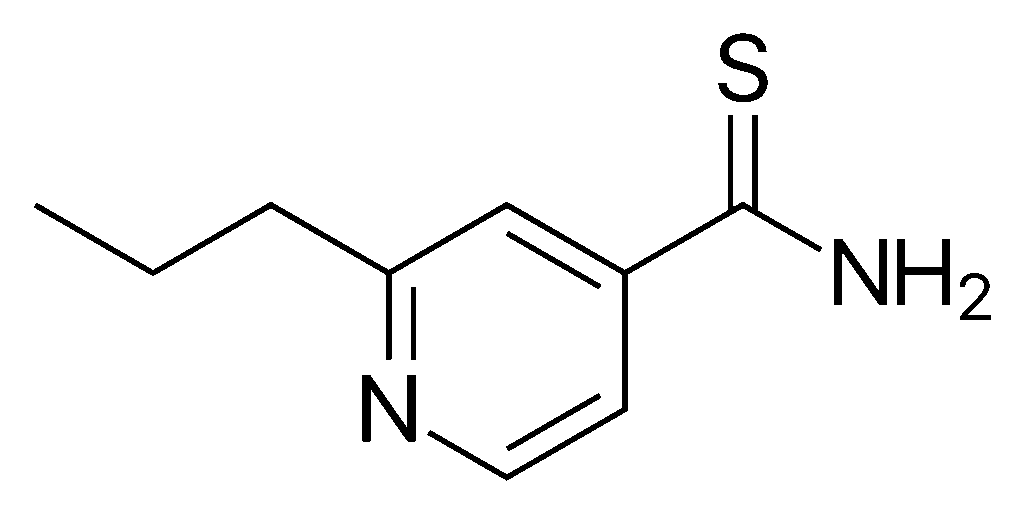
| IUPAC_name = 2-propylpyridine-4-carbothioamide
|
| IUPAC_name = 2-propylpyridine-4-carbothioamide
|
||
|
| image = Protionamide.svg
|
| image = Protionamide.svg
|
||
|
| alt =
|
|||
|
| image2 = Prothionamide 3D ball.png
|
| image2 = Prothionamide 3D ball.png
|
||
|
| alt2 =
|
|||
|
<!–Clinical data–>
|
<!–Clinical data–>
|
||
| Line 12: | Line 16: | ||
|
| Drugs.com = {{drugs.com|international|prothionamide}}
|
| Drugs.com = {{drugs.com|international|prothionamide}}
|
||
|
| pregnancy_AU = <!– A / B1 / B2 / B3 / C / D / X –>
|
| pregnancy_AU = <!– A / B1 / B2 / B3 / C / D / X –>
|
||
|
| pregnancy_US = <!– A / B / C / D / X –>
|
|||
|
| pregnancy_category =
|
| pregnancy_category =
|
||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
|
| legal_AU = <!– Unscheduled / S2 / S3 / S4 / S5 / S6 / S7 / S8 / S9 –>
|
| legal_AU = <!– Unscheduled / S2 / S3 / S4 / S5 / S6 / S7 / S8 / S9 –>
|
||
|
| legal_CA = <!– / Schedule I, II, III, IV, V, VI, VII, VIII –>
|
| legal_CA = <!– / Schedule I, II, III, IV, V, VI, VII, VIII –>
|
||
| Line 19: | Line 26: | ||
|
| legal_US = <!– OTC / Rx-only / Schedule I, II, III, IV, V –>
|
| legal_US = <!– OTC / Rx-only / Schedule I, II, III, IV, V –>
|
||
|
| legal_status =
|
| legal_status =
|
||
| ⚫ | |||
|
<!–Pharmacokinetic data–>
|
<!–Pharmacokinetic data–>
|
||
| Line 31: | Line 37: | ||
|
| CAS_number_Ref = {{cascite|correct|??}}
|
| CAS_number_Ref = {{cascite|correct|??}}
|
||
|
| CAS_number = 14222-60-7
|
| CAS_number = 14222-60-7
|
||
| ⚫ | |||
| ⚫ | |||
|
| PubChem = 666418
|
| PubChem = 666418
|
||
|
| DrugBank_Ref = {{drugbankcite|correct|drugbank}}
|
| DrugBank_Ref = {{drugbankcite|correct|drugbank}}
|
||
| Line 51: | Line 55: | ||
|
”’Protionamide”’ (or ”’prothionamide”’) is a drug used in the treatment of [[tuberculosis]] and [[leprosy]].<ref name=”pmid17227913″>{{cite journal |vauthors=Wang F, Langley R, Gulten G, etal |title=Mechanism of thioamide drug action against tuberculosis and leprosy |journal=J. Exp. Med. |volume=204 |issue=1 |pages=73–8 |date=January 2007 |pmid=17227913 |pmc=2118422 |doi=10.1084/jem.20062100 |url=http://www.jem.org/cgi/pmidlookup?view=long&pmid=17227913}}</ref><ref name=”pmid16525107″>{{cite journal |vauthors=Fajardo TT, Guinto RS, Cellona RV, Abalos RM, Dela Cruz EC, Gelber RH |title=A clinical trial of ethionamide and prothionamide for treatment of lepromatous leprosy |journal=Am. J. Trop. Med. Hyg. |volume=74 |issue=3 |pages=457–61 |date=March 2006 |doi=10.4269/ajtmh.2006.74.457 |pmid=16525107 |s2cid=21415032 |url=http://www.ajtmh.org/cgi/pmidlookup?view=long&pmid=16525107|doi-access=free |url-access=subscription }}</ref>
|
”’Protionamide”’ (or ”’prothionamide”’) is a drug used in the treatment of [[tuberculosis]] and [[leprosy]].<ref name=”pmid17227913″>{{cite journal |vauthors=Wang F, Langley R, Gulten G, etal |title=Mechanism of thioamide drug action against tuberculosis and leprosy |journal=J. Exp. Med. |volume=204 |issue=1 |pages=73–8 |date=January 2007 |pmid=17227913 |pmc=2118422 |doi=10.1084/jem.20062100 |url=http://www.jem.org/cgi/pmidlookup?view=long&pmid=17227913}}</ref><ref name=”pmid16525107″>{{cite journal |vauthors=Fajardo TT, Guinto RS, Cellona RV, Abalos RM, Dela Cruz EC, Gelber RH |title=A clinical trial of ethionamide and prothionamide for treatment of lepromatous leprosy |journal=Am. J. Trop. Med. Hyg. |volume=74 |issue=3 |pages=457–61 |date=March 2006 |doi=10.4269/ajtmh.2006.74.457 |pmid=16525107 |s2cid=21415032 |url=http://www.ajtmh.org/cgi/pmidlookup?view=long&pmid=16525107|doi-access=free |url-access=subscription }}</ref>
|
||
|
Protionamide is a therapeutic alternative on the [[WHO Model List of Essential Medicines|World Health Organization’s List of Essential Medicines]].<ref name=”WHO24th”>{{cite book | vauthors = ((World Health Organization)) | title = The selection and use of essential medicines, 2025: WHO Model List of Essential Medicines, 24th list | year = 2025 | hdl = 10665/382243 | author-link = World Health Organization | publisher = World Health Organization | location = Geneva | hdl-access=free }}</ref>
|
|||
|
==References==
|
==References==
|
||
| Line 56: | Line 62: | ||
|
{{Antimycobacterials}}
|
{{Antimycobacterials}}
|
||
|
{{Portal bar | Medicine}}
|
|||
|
{{Authority control}}
|
|||
|
[[Category:Disubstituted pyridines]]
|
[[Category:Disubstituted pyridines]]
|
||
|
[[Category:Thioamides]]
|
[[Category:Thioamides]]
|
||
|
[[Category:Anti-tuberculosis drugs]]
|
[[Category:Anti-tuberculosis drugs]]
|
||
|
[[Category:World Health Organization essential medicines]]
|
|||
|
{{antiinfective-drug-stub}}
|
{{antiinfective-drug-stub}}
|
||